Importance Of Serial Dilution In Serological Tests

Serological non-response or serofast status was defined as no more than a 2-fold (1 dilution) increase or decrease from baseline. As specified in the protocol, those participants defined as treatment failure or serological non-response were retreated at 6 months with benzathine penicillin or doxycycline, if penicillin allergic. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. Serial Dilution: Importance and Aplication. Serial dilution is a simple yet efficient technique to determine the. Importance Of Serial Dilution In Serology. Oct 19, 2011 Serial dilution is a simple yet efficient technique to determine the number of cells or organisms in a concentrated sample. First, take a portion of the sample and does serial dilution on it. Repeat the steps until the cells can be observed under the microscope when the.
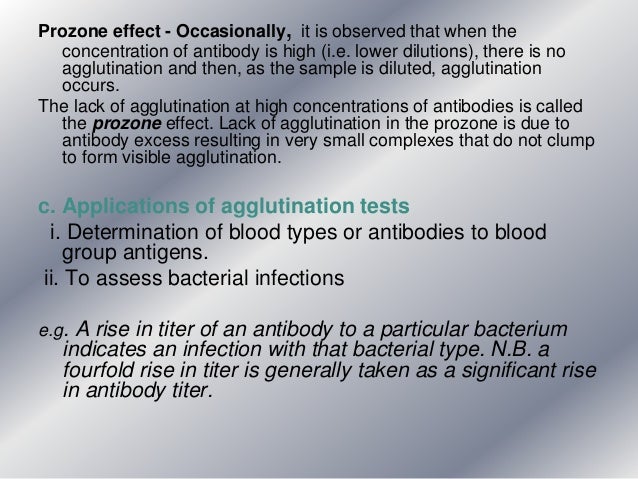
An antibody titer is a measurement of how much antibody an organism has produced that recognizes a particular epitope, expressed as the inverse of the greatest dilution (in a serial dilution) that still gives a positive result. ELISA is a common means of determining antibody titers.
For example, the indirect Coombs test detects the presence of anti-Rh antibodies in a pregnant woman's blood serum. A patient might be reported to have an 'indirect Coombs titer' of 16. This means that the patient's serum gives a positive indirect Coombs test at any dilution down to 1/16 (1 part serum to 15 parts diluent). At greater dilutions the indirect Coombs test is negative. If a few weeks later the same patient had an indirect Coombs titer of 32 (1/32 dilution which is 1 part serum to 31 parts diluent), this would mean that she was making more anti-Rh antibody, since it took a greater dilution to abolish the positive test.
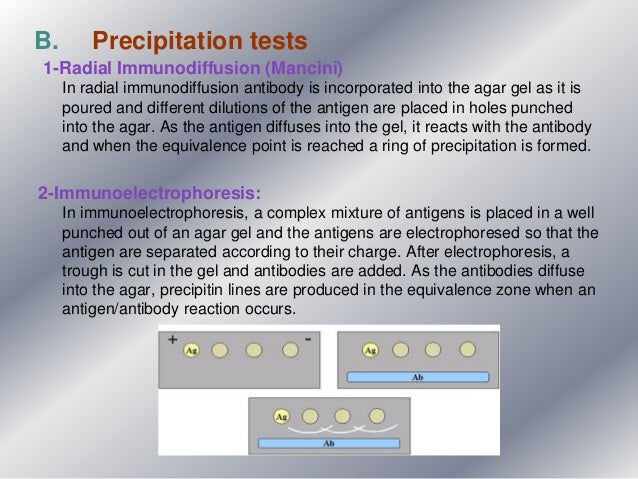
Many traditional serological tests such as hemagglutination or complement fixation employ this principle. Such tests can typically be read visually, which makes them fast and cost-effective in a 'low-tech' environment. The interpretation of serological titers is guided by reference values that are specific to the antigen or antibody in question; a titer of 1:32 may be below the cut-off for one test but above for another.
A common design for estimating theconcentrations of compounds in biological samples is the serialdilution assay, in which measurements are taken at severaldifferent dilutions of a sample, giving several opportunities foran accurate measurement. Curren tly, serial dilution is a standardtool in the fields of toxicology and immunology.
Serial dilution helps to choose a dilution whichis relevant to our experiment.
Often the standard which is given to you in thelab is far to strong for the experiment and it needs to be diluted.But equally the equipment has a detection limit so we can't diluteit to much, or if it is too diluted the experiment might notwork.
What is the differences between a streak plate technique and a serial dilution technique?
What are the advantages of dilution plate method?
Why doing serial dilution?
What tests determine an unknown bacteria?
What is serial dilution?
What is the purpose of adding water in alcohol?
What is disadvantages of serial dilution?
What is the purpose of using streak plate technique?
Use of test tube?
How can you prepare solution 0.2 ppm from 1000 ppm?
How do you prepare 1 is to 100 dilution in small volume?
What are the method of estimating microbial population in soil?
What is the purpose of a serial mouse?
Definition of dilution and dilution factor?
What is the effect of dilution on viscosity of oil?
12 Distinguish between dilution and dilution factor?
Distinguish between dilution and dilution factor?
What is infinite dilution?
What does 0 percent dilution mean?
What is the equation for a dilution?
How does the number of moles of solute before a dilution compare with the number of solute after the dilution?
Why do ABBA special edition have serial numbers?
What is dilution factor?
What is the difference between dilution and diffusion?
How does dilution affect concentration of a solution?
What is another term for dilution?
What is helium dilution technique?
What is titer?